The Basics: Isotopic Fingerprints
- Isotope Symbol Calculator
- Isotopes Have Different Number Of
- Isotopes Have Different Numbers Of
- Isotopes Have
- Isotopes Have Same Mass
- Isotopes Have Similar
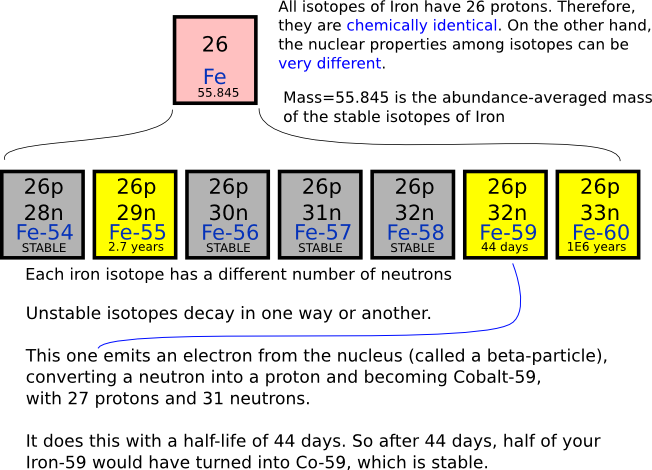
Hydrogen has three naturally occurring isotopes: 1 H (protium), 2 H (deuterium), and 3 H (tritium). Other highly unstable nuclei (4 H to 7 H) have been synthesized in the laboratory, but do not occur in nature. The most stable radioisotope of hydrogen is tritium, with a half-life of 12.32 years. An isotope is one of several kinds of atoms of the same element that have different masses. These atoms have the same number of protons in their nuclei, but different numbers of neutrons, and therefore different mass numbers.

 By Lauren Shoemaker
By Lauren ShoemakerAugust, 2010
Lauren is a mathematics and biology double major at Colorado College. Shethanks NOAA ESRL's Carbon Cycle Greenhouse Gases group, and the Stable Isotope Laboratory and the Radiocarbon Laboratory, both at INSTAAR (University of Colorado), for their help in creating this website.
This project was made possible through the National Oceanic and Atmospheric Administration's Ernest F. Hollings Scholarship Program.
A Mixture of Knowledge and Questions
Ever since the early 1960s, atmospheric scientists have known that carbon dioxide levels in the atmosphere are on the rise. We know that this increase is caused by burning of fossil fuels - coal, oil and natural gas - which emit carbon dioxide into the air. Yet only about half of the carbon dioxide produced each year from fossil fuels remains in the atmosphere. The rest is absorbed by the oceans and land plants.
Even with this wealth of knowledge, scientists have more to discover about carbon dioxide levels in our atmosphere and its interactions with the oceans and land.
- How much of the carbon dioxide in the air comes from fossil fuels?
- How is this changing?
- Where in the world is the carbon dioxide from fossil fuels coming from?
- How much is coming from each region?
- How much of the added CO2 goes into the oceans, and how much into the biosphere?
- Which parts of the biosphere are absorbing CO2, and why?
- Are some parts of the biosphere releasing CO2 to the atmosphere?
- How much carbon dioxide is added to the atmosphere from deforestation?
- Is this offset by increased plant growth in other parts of the terrestrial biosphere?
- How much carbon dioxide is being absorbed into the ocean?
- Which parts of the ocean are absorbing the carbon dioxide?
- What other effects may this have, such as the ocean becoming more acidic 1?
Answering these questions will help us to understand what might happen in the future as CO2 levels continue to rise. Will these surface reservoirs “fill up” and no longer take up the extra CO2, leading to an even faster rise in CO2 in the atmosphere? Could we discover ways to increase the amount of CO2 taken up by these surface reservoirs, and thus reduce the amount of CO2 in the atmosphere?
To answer these questions, scientists at NOAA's Carbon Cycle and Greenhouse Gases Group use measurements of greenhouse gases and their isotopes.
The Carbon Cycle
Isotope Symbol Calculator
To truly understand the role of carbon dioxide in our atmosphere, and how we, as humans, may be altering our atmosphere, we have to look at the entire carbon cycle. The carbon cycle describes and quantifies the movement of carbon through different reservoirs on Earth.

The main reservoirs that climate scientists study are:
- the atmosphere
- the terrestrial biosphere (life on land)
- the ocean
- fossil fuels (ancient plants that have been buried underground for millions of years)
Isotopes Have Different Number Of
The last two reservoirs depicted in the diagram above–the terrestrial biota and the ocean–can be further classified as net sources or net sinks of atmospheric carbon dioxide. While sources are components that add carbon dioxide to the atmosphere over a long period of time, sinks take in, or sequester, atmospheric carbon dioxide. Note that scientists add the term “net” before source or sink. This is because carbon dioxide is exchanged both ways in the terrestrial biosphere and the ocean. In other words, using the ocean as an example, carbon dioxide is constantly both entering and exiting the ocean. Using the term “net sink” tells us that more carbon dioxide enters the ocean than leaves the ocean. The same is true for the terrestrial biosphere.
Isotopes are the Key
How can we distinguish between the different sources and sinks of carbon dioxide? Carbon dioxide, or CO2, contains the key piece of information within the carbon atoms themselves. Although it may seem that a carbon atom is just the same as every other carbon atom out there (perhaps they appear to all be clones of each other–where each looks and acts exactly the same), this is not the case.
In fact there are three isotopes of carbon atoms - all three react the same way in chemical reactions–the only chemical difference between them is that they have slightly different masses. The heaviest is carbon-14 (which, in the scientific world, is written as 14C), followed by carbon-13 (13C), and the lightest, most common carbon-12 (12C). Different carbon reservoirs “like” different isotopes, so the relative proportion of the three isotopes is different in each reservoir - each has its own, identifying, isotopic fingerprint. By examining the isotopic mixture in the atmosphere, and knowing the isotopic fingerprint of each reservoir, atmospheric scientists can determine how much carbon dioxide is coming and going from each reservoir, making isotopes an ideal tracer of sources and sinks of carbon dioxide.
Isotopes Have Different Numbers Of
As an example of these isotopic fingerprints, and how they can help scientists, consider this: fossil fuels do not contain 14C. By studying how the concentration of 14C has changed in the atmosphere, scientists have determined that the atmospheric increase in carbon dioxide is dominated by fossil fuel emissions. While terrestrial plants “dislike” 13C, ocean exchange does not prefer 12C or13C. This creates a difference in the relative ratio of terrestrial versus oceanic uptake of atmospheric carbon dioxide isotopes.
Isotopes Have
The key difference between parent and daughter isotopes is that a parent isotope undergoes radioactive decay to form a daughter isotope.
The terms parent and daughter isotopes come under the category of isotopes of chemical elements. Isotopes are different forms of a single chemical element. Therefore, isotopes have the same atomic number but different mass numbers because they differ from each other according to the number of neutrons present in their atomic nuclei. Among the isotopes of a chemical element, some or all of the isotopes are radioactive. They undergo radioactive decay to form different chemical elements.
CONTENTS
1. Overview and Key Difference
2. What are Parent Isotopes
3. What are Daughter Isotopes
4. Side by Side Comparison – Parent vs Daughter Isotopes in Tabular Form
5. Summary
What are Parent Isotopes?
Parent isotopes are the isotopes of a particular chemical element that can undergo radioactive decay to form a different isotope from a different chemical element. During this radioactive decay, these isotopes release decay particles such as alpha, beta and gamma rays. A parent isotope is the beginning of a decay chain. A decay chain is a series of radioactive decaying reactions that takes place starting from a single isotope (the parent isotope).
An example of a parent isotope is Uranium. It can undergo radioactive decay to form thorium via alpha decay. The time taken by a parent isotope to decay into a daughter isotope can vary from one isotope to another; sometimes the nature of the parent isotope determines the time and sometimes the nature of the daughter isotope formed from decay process determines the time.
What are Daughter Isotopes?
Daughter isotopes are the products of radioactive decay of parent isotopes. Sometimes reactions give stable daughter isotopes, but most of the times they are unstable and radioactive, which leads to the progression of decay chains. Moreover, daughter isotopes undergo radioactive decay to form daughter isotopes of their own. These are called as granddaughter isotopes (daughter isotopes of the daughter isotopes).
Figure 02: A Decay Chain
For example, thorium is a daughter isotope that forms from the radioactive decay of uranium. Some other terms that we can use to name daughter isotopes are daughter product, decay product, daughter nuclide, radio-daughter, etc.
What is the Difference Between Parent and Daughter Isotopes?
The terms parent and daughter isotopes come under the category of isotopes of chemical elements. Isotopes are different forms of a single chemical element. Most isotopes are radioactive. The key difference between parent and daughter isotopes is that a parent isotope undergoes radioactive decay to form a daughter isotope. An example of a parent isotope is Uranium. It can undergo alpha decay and form thorium. Therefore, thorium is the daughter isotope of this reaction. Thorium can undergo further decay, which leads to a decay chain.
Most of the times, daughter isotopes are unstable and undergo further decay. But, sometimes they are stable products. However, the parent isotopes are always unstable isotopes. Furthermore, it is also important to note that the daughter isotope is always a different chemical element than the parent isotope.
The below infographic summarizes the difference between parent and daughter isotopes.
Isotopes Have Same Mass
Summary – Parent vs Daughter Isotopes

The terms parent and daughter isotopes come under the category of isotopes of chemical elements. Isotopes are different forms of a single chemical element. Most isotopes are radioactive. Parent isotopes are the isotopes of a particular chemical element that can undergo radioactive decay to form a different isotope from a different chemical element. Daughter isotopes, on the other hand, are the products of radioactive decay of parent isotopes. So, this is the key difference between parent and daughter isotopes.
Reference:
1. Helmenstine, Anne Marie. “Daughter Isotope Definition – Chemistry Glossary.” ThoughtCo, Jan. 12, 2020, Available here.
Image Courtesy:
1. “NuclearReaction” By Kjerish – Own work (CC BY-SA 4.0) via Commons Wikimedia
2. “Decay Chain Thorium” By BatesIsBack– (CC BY-SA 3.0) via Commons Wikimedia
Isotopes Have Similar
Related posts:
