Atomic Mass and Nuclear Binding Energy for Ar-32 (Argon)
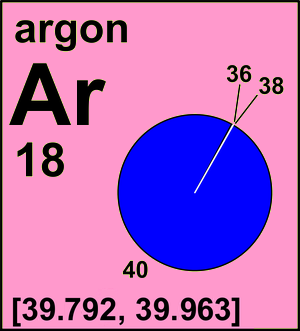
Argon is a chemical element with atomic number 18 which means there are 18 protons and 18 electrons in the atomic structure. The chemical symbol for Argon is Ar. Atomic Mass of Argon Atomic mass of Argon is 39.948 u. Argon constitutes 1.288 percent of the atmosphere by weight and 0.934 percent by volume and is found occluded in rocks. Although the stable isotopes argon-36 and argon-38 make up all but a trace of this element in the universe, the third stable isotope, argon-40, makes up 99.60 percent of the argon found on Earth. (Argon-36 and argon-38 make up. Since in the normal production process, argon was used as a protecting gas, the possibility of suffocation in an argon atmosphere was investigated. This was rendered more difficult because of the natural content of 0.93 vol.% argon in air and since the excessive argon could have been removed by the resuscitation attempts. Argon is a chemical element in the 18 group of the periodic table. Know the Uses of Argon, Chemical Properties of Argon, Atomic Mass of Argon, & more at BYJU'S.
Abstract
This document is part of the Supplement containing the complete sets of data of Subvolume A `Nuclei with Z = 1 - 54' of Volume 22 `Nuclear Binding Energies and Atomic Masses' of Landolt-Börnstein - Group I `Elementary Particles, Nuclei and Atoms'. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Ar-32 (Argon, atomic number Z = 18, mass number A = 32).

- atomic mass;
- mass excess;
- nuclear binding energy;
- nucleon separation energy;
- Q-value;
- nucleon residual interaction parameter
Atomic Mass and Nuclear Binding Energy for Ar-32 (Argon)

Abstract
This document is part of the Supplement containing the complete sets of data of Subvolume A `Nuclei with Z = 1 - 54' of Volume 22 `Nuclear Binding Energies and Atomic Masses' of Landolt-Börnstein - Group I `Elementary Particles, Nuclei and Atoms'. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Ar-32 (Argon, atomic number Z = 18, mass number A = 32).
Argon Atomic Mass And Number
Symbol For Argon
Atomic Number Of Argon
- atomic mass;
- mass excess;
- nuclear binding energy;
- nucleon separation energy;
- Q-value;
- nucleon residual interaction parameter
